cn- lewis structure
Lewis diagram of the cyanide ion CN We can draw Lewis structures for polyatomic ions ions containing multiple atoms using the same stepwise procedure as for. CN- cyanide ion lewis structure has one Carbon atom C and one Nitrogen atom N which contain a triple bond between them.
 |
| Write The Lewis Structure For Cn 8 Ion |
On the opposite sides on each atom are two dots for.
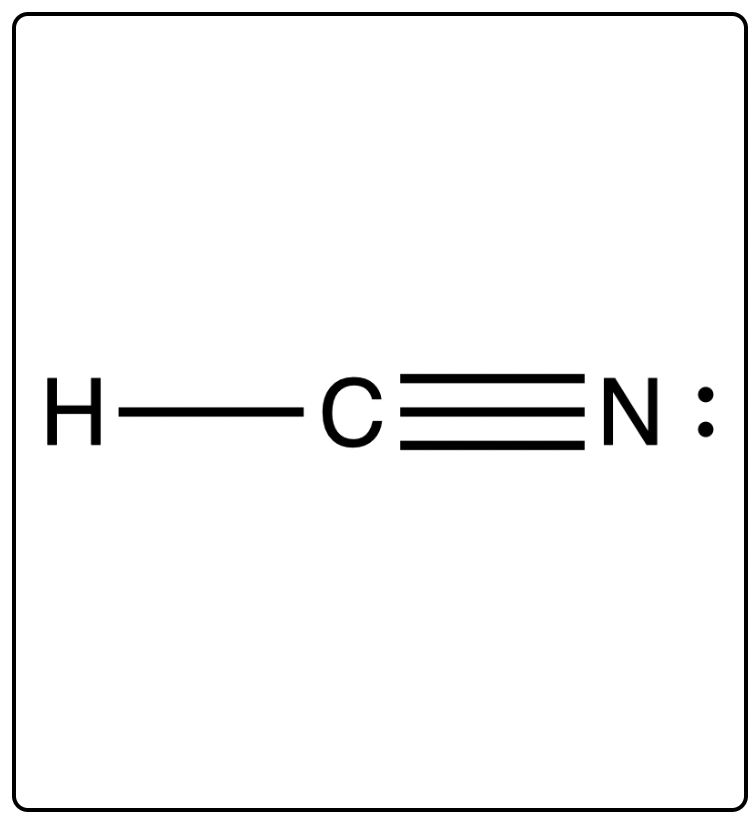
. The Lewis Dot structure of Cyanide ion starts with a C atom connected to a N atom with three dashes. Count the total valance electrons For drawing Lewis diagram we need to determine the total valence electrons on all constituent atoms and. Lewis Dot Structure of CN- Cyanide Ion 150923 views Oct 11 2011 I quickly take you through how to draw the Lewis Structure of CN- CyanideIon. Thus CN- has 10 valence electrons.
In the lewis structure of CN there is a triple bond between the carbon and nitrogen atom and. In this video well go through the steps to write the CN- Lewis Dot Structure Cyanide ionFor the CN- structure use the periodic table to find the total nu. Drawing the Lewis Dot Structure for Cyanide. The lewis structure of CN contains a triple bond between the carbon atom and nitrogen atom and both the atoms have one lone pair.
I also go over the hybridizatio 354. Triple BondThis video shows how to draw the lewis dot structure of Cyanide which involves using a triple bond. In the lewis structure of CH 3 CN there is a single bond between the two. CN- Lewis Structure The Lewis structure of any molecule is a pictorial representation of the arrangement of atoms and electrons in the.
Draw the molecule by placing atoms on the grid and connecting them with bonds. Draw Lewis structure for CNCN. CN Lewis Structure Molecular Geometry Hybridization Polarity and MO Diagram CN is known as cyanide which exists as a pseudohalide anion. CN- Lewis Structure CN cyanide has one carbon atom and one nitrogen atom.
It is a chemical formula for CyanideTo understand the Lewis structure of CN-. CH 3 CN acetonitrile has two carbon atoms three hydrogen atoms and one nitrogen atom. There is 1 lone pair on both the Carbon atom C. Draw Lewis Structure of Cyanide Ion CN in 6 Easy Steps The Lewis structure merely takes into account the electrons present in the valence shell neglecting the inner shells.
Plus there is a negative -1 charge on the carbon. Hey GuysIn this video we are going to learn about the Lewis structure of CN-. It belongs to the cyano group and. Lewis structure of CN.
Steps for drawing Lewis dot structure of CN 1. CN- lewis structure showing two possible resonances The left hand side resonance structure has a triple bond between C and N and also has a lone electron pair on each both atoms. For drawing the Lewis structure for a compound we must first calculate the total number of valence electrons by adding up the valence electrons of all the participating atoms. Include all lone pairs of electrons and nonbonding electrons.
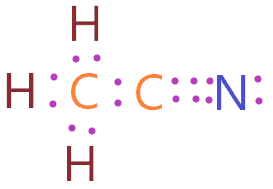 |
| Ch3cn Lewis Structure Molecular Geometry Bond Angle Polarity Electrons |
 |
| Fall 2011 Final Exam Answers |
 |
| Cn Lewis Structure How To Draw The Dot Structure For The Cn Youtube |
 |
| Barium Cyanide Wikipedia |

Posting Komentar untuk "cn- lewis structure"